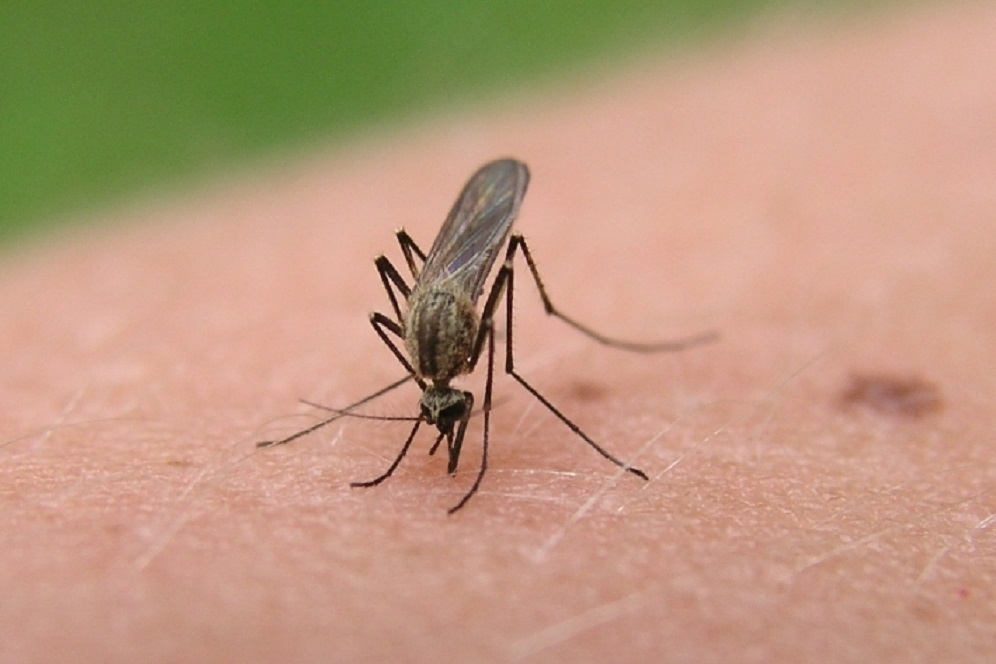
The National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, has launched a Phase 1 clinical trial to test an investigational vaccine intended to provide broad protection against a range of mosquito-transmitted diseases, such as Zika, malaria and West Nile fever.
The AGS-v candidate is designed to trigger an immune response to mosquito saliva rather than to a specific virus or parasite carried by mosquitoes. The test vaccine contains four synthetic proteins from mosquito salivary glands, officials said.
The proteins are designed to induce antibodies in a vaccinated individual and to cause a modified allergic response that can prevent infection when a person is bitten by a disease-carrying mosquito.
The investigational vaccine was developed by the London-based pharmaceutical company SEEK, which has since formed a joint venture with hVIVO in London. The consulting group Halloran has provided regulatory advice to both companies.
The study is being conducted at the NIH Clinical Center in Bethesda, Maryland. The clinical trial is expected to enroll up to 60 healthy adults ages 18 to 50. Participants will be randomly assigned to receive one of three vaccine regimens.
The study is expected to be completed by summer 2018.