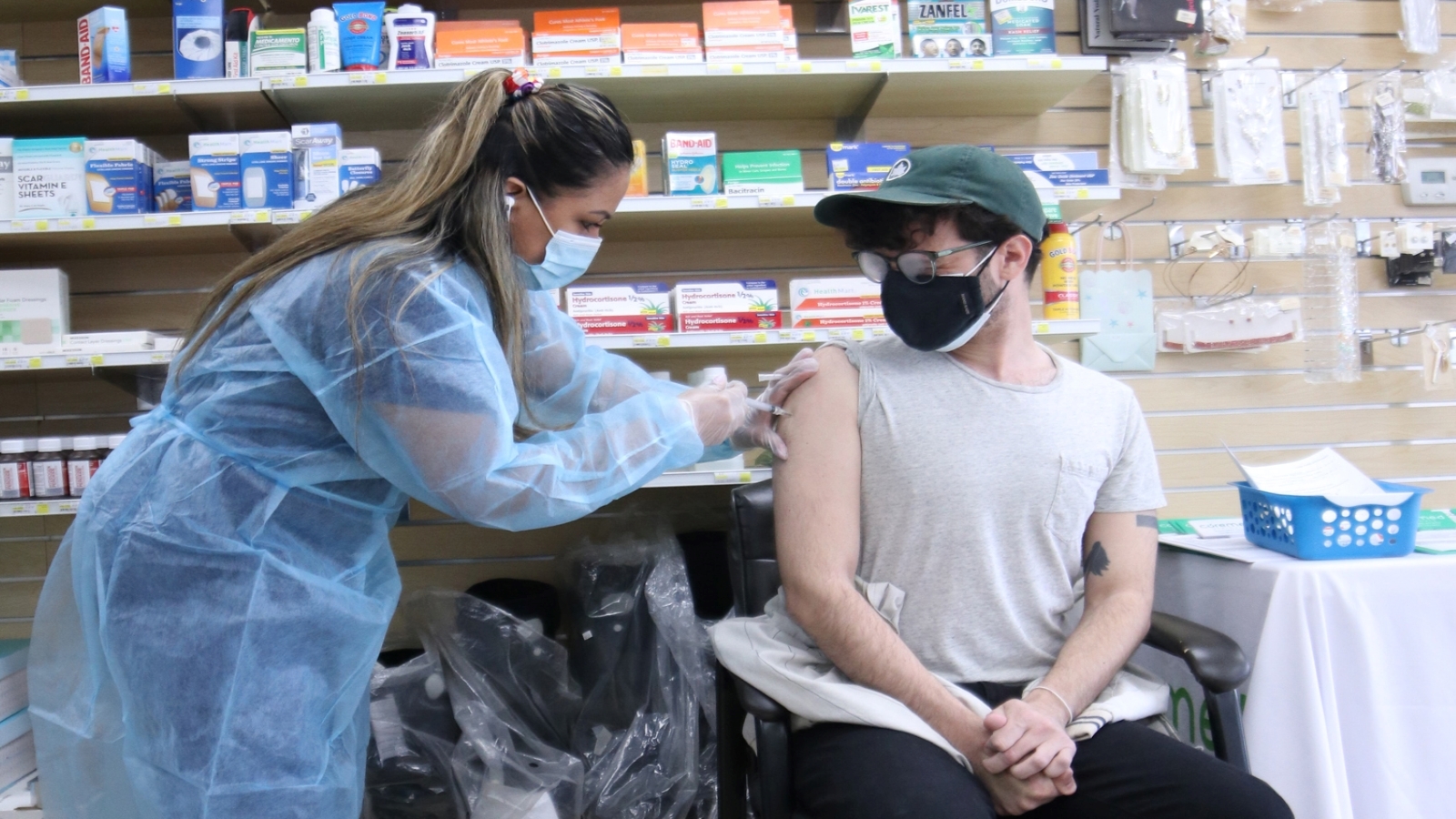
Pfizer’s coronavirus vaccine has received the U.S. Food and Drug Administration’s full approval for use in people ages 16 and older, as of Aug. 23.
Pfizer’s breakthrough includes being the first to have been granted approval for a COVID-19 vaccine.
"The FDA's approval of this vaccine is a milestone as we continue to battle the COVID-19 pandemic,” acting FDA Commissioner Janet Woodcock said, according to U.S. News and World Report. “While this and other vaccines have met the FDA's rigorous, scientific standards for emergency use authorization, as the first FDA-approved COVID-19 vaccine, the public can be very confident that this vaccine meets the high standards for safety, effectiveness and manufacturing quality the FDA requires of an approved product."
Now under the trade name, Comirnaty, Pfizer and BioNTech are allowed to market their vaccine directly to consumers, according to U.S. News & World Report.
The vaccine is still available to 12- to 15-year-olds under emergency use authorization.
According to U.S. News & World Report, more than 51% of Americans are now fully vaccinated against COVID-19. Public health officials hope that with the full approval of the vaccine, more people will have confidence in its safety and be encouraged to get their shot as the delta variant has surged throughout the country. They are also hoping that there more businesses will mandate the fully approved vaccine for employees.