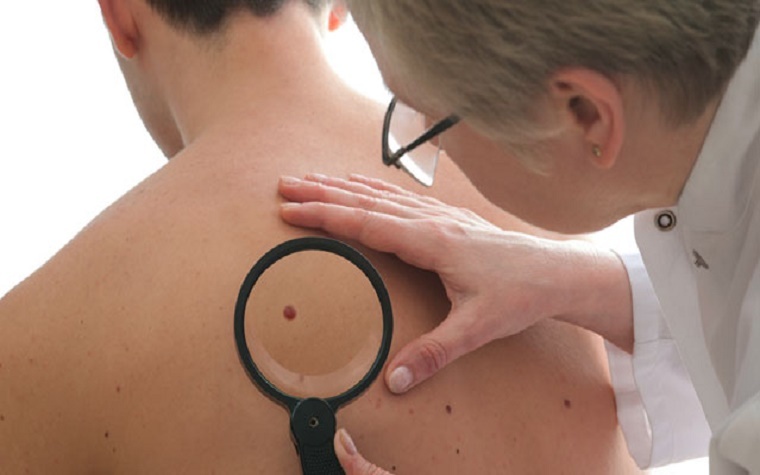
Opdivo, a medication effective for certain forms of high-risk skin cancer, has earned approval from the European Medicines Agency for expanded use in patients with advanced melanoma who have had tumors surgically removed, Bristol-Myers Squibb, the drug's manufacturer, said in a release.
The approval of the drug’s Type II variation application will allow Opdivo to treat Stage III melanoma in patients who ahve a near 70 percent chance of the cancer recurring , the release said.
The European regulators made the decision to allow Opdivo’s expanded indication after positive results from a Phase 3 clinical trial were presented in September at the European Society for Medical Oncology Congress in Madrid. Other components of the study are still underway, the release said.
Bristol-Myers Squibb developed Opdivo as part of its ongoing efforts to combat tough cases of cancer through the use of immuno-oncology, which marshals the body’s own immune system to battle the disease, the release said.