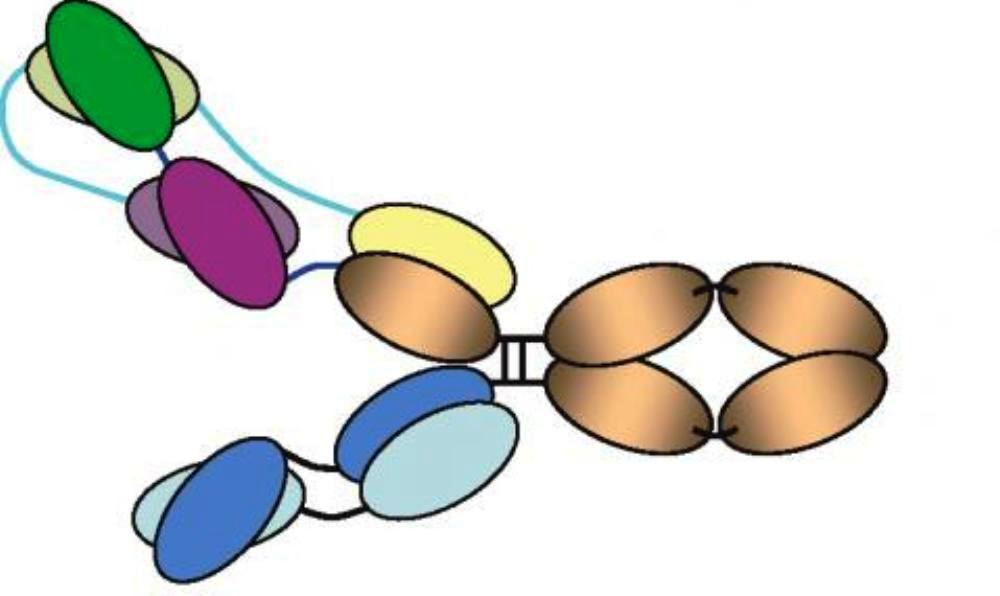
National Institutes of Health (NIH) and Sanofi researchers recently found that a laboratory-generated, three-pronged antibody protects monkeys from HIV-type infection better than the natural antibodies it's based on, according to an announcement.
The findings have spurred scientists to design early-phase clinical trials of this “trispecific” antibody. By binding to three locations on the HIV virus, this antibody may stand a better chance of intervening with the virus than single antibodies. Already shown to thwart the progression of the disease — called SHIV in monkeys — the trispecific version may also have a potential for preventing or treating HIV in humans.
“Combinations of antibodies that each bind to a distinct site on HIV may best overcome the defenses of the virus in the effort to achieve effective antibody-based treatment and prevention,” Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases (NIAID), part of NIH, said in the announcement. “The concept of having a single antibody that binds to three unique sites on HIV is certainly an intriguing approach for investigators to pursue.”
Team members at NIAID’s Vaccine Research Center collaborated with Sanofi scientists, testing different combinations of bispecific and trispecific antibodies until they found the most effective combination. They then administered infusions to groups of monkeys prior to exposure to SHIV strains. Not one monkey receiving the trispecific antibody contracted an infection.
Researchers hope to develop similar prototypes for other infectious diseases in addition to HIV; Sanofi and NIAID plan a clinical trial for late 2018.