Dupixent (dupilumab), an injectable drug from Sanofi and Regeneron Pharmaceuticals Inc. used to treat moderate to severe atopic dermatitis (AD), has been approved by the Food and Drug Administration (FDA).
Dupixent is the only biologic approved for AD, according to a press release. It can be used when the disease doesn't respond adequately to prescription topical or when topical medications are not appropriate.
"People with moderate-to-severe atopic dermatitis cope with intense, sometimes unbearable symptoms that can impact them for most of their lives," National Eczema Association President and CEO Julie Block said in the release. "To date, there have been few options available to treat people with moderate-to-severe atopic dermatitis who have uncontrolled disease. That's why today's approval of Dupixent is so important for our community. Now we have a treatment that is expected to help address patients suffering from this devastating disease."
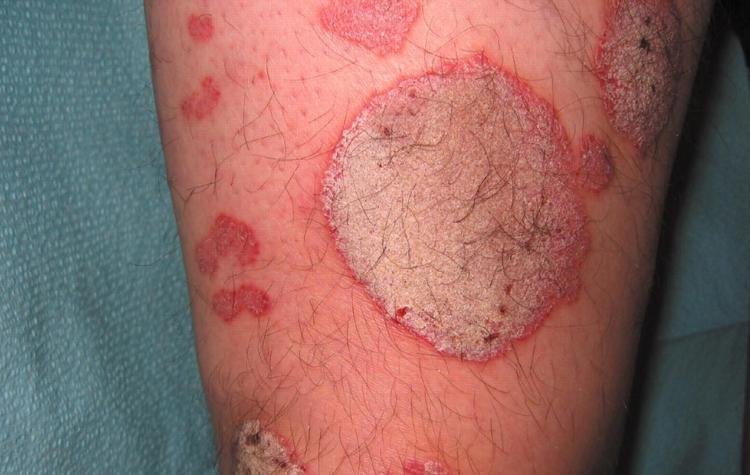
AD is a chronic inflammatory disease. It is the most common form of eczema. The red, itching, crusting or oozing rashes may cover most of the patient's body. The itching alone can be debilitating.
Dupixent is available in a pre-filled syringe and may be self-applied every other week. It is designed to inhibit two key proteins, IL-4 and IL-13, and reduce AD's underlying inflammation. Patients can also use topical corticosteroids in conjunction with Dupixent.