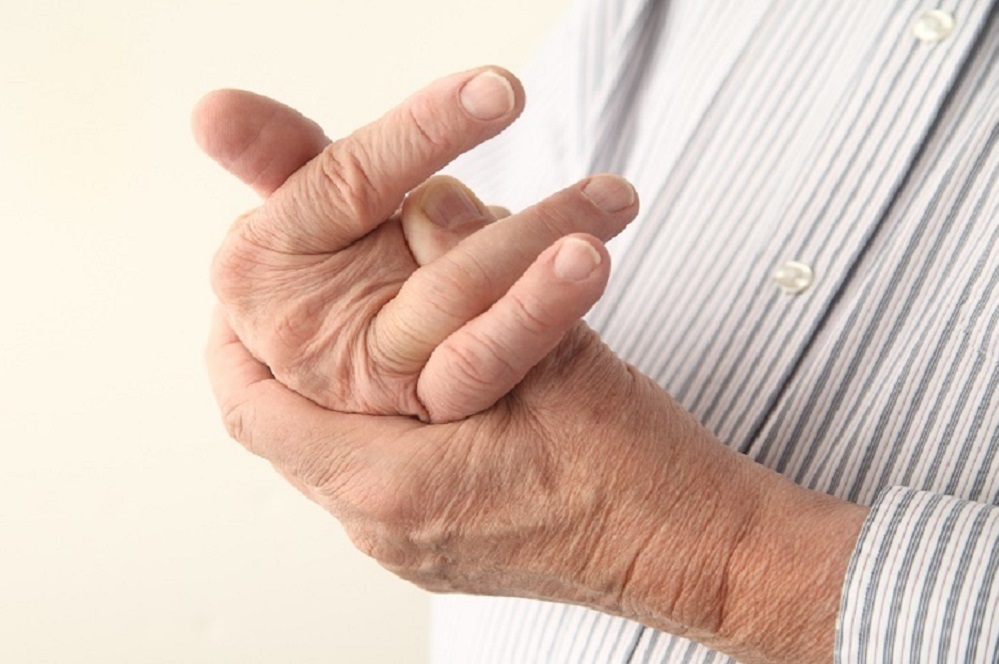
Patients taking the rheumatoid arthritis drug baricitinib showed greater improvement than those taking adalimumab, sold under the brand name Humira, over the same period, Eli Lilly and Company reported recently.
The results, published in the New England Journal of Medicine, focused on phase 3 of the efficacy of baricitinib in treating rheumatoid arthritis. Patients taking baricitinib started achieving ACR50 and ACR70 responses as early as week eight and continuing through week 52 -- an improvement of between 50 percent and 70 percent compared with adalimumab.
Eli Lilly had previously reported that baricitinib had met its primary objective of outperforming a placebo after 12 weeks of treatment.
"This is an exciting time for rheumatology, with potential new treatments for rheumatoid arthritis on the horizon,” Dr. Peter Taylor, study author, and Norman Collisson, chair of Musculoskeletal Sciences in the Nuffield Department of Orthopedics, Rheumatology and Musculoskeletal Sciences at the University of Oxford, said.
Taylor and Collisson said this was the first phase 3 trial that indicated that a once-daily, oral treatment showed a significant improvement compared with a current standard of care.
The study was conducted by Eli Lilly and Company and Incyte Corp.