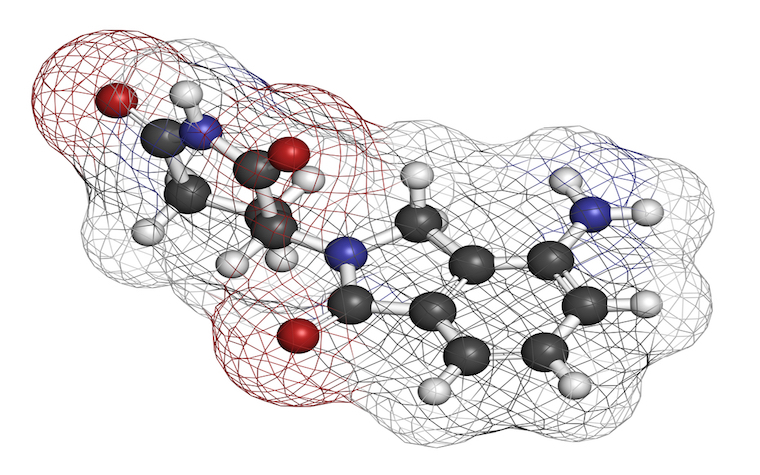
Japanese patients living with blood and bone marrow cancer, or multiple myeloma, may soon have another option for treatment with the Japanese Ministry of Health, Labor and Welfare's approval of ixazomib, a product of Takeda Pharmaceutical Co. Ltd., as an Orphan Drug.
Orphan Drug designation is granted to therapies found in studies to be effective in the treatment of rare diseases. Ixazomib is indicated for the treatment of patients whose multiple myeloma has relapsed or is refractory. It is an investigational oral proteasome inhibitor.
Multiple myeloma affects the bone marrow's plasma cells. The disease is marked by the multiplication of monoclonal plasma (myeloma) cells, which become cancerous. It can spread to many bones in the body and cause complications such as compression fractures, lytic bone lesions and pain -- as well as problems in the immune system, kidneys and red blood cells.
About 14,000 patients in Japan are currently living with multiple myeloma.