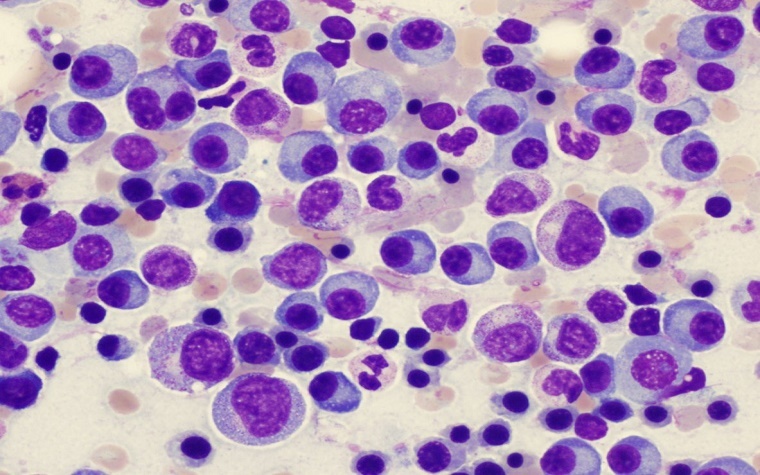
With the U.S. Food and Drug Administration's (FDA) approval of Ninlaro, patients living with multiple myeloma, a blood cancer of the bone marrow, have a new treatment option. The approval allows Ninlaro (ixazomib) to be used in combination with two other therapies for multiple myeloma patients who have tried at least one unsuccessful therapy.
Some side effects of Ninlaro have been reported, including diarrhea, constipation, low blood platelet count, numbness and pain in the hands and feet as a result of nerve damage, nausea, swelling caused by fluid retention under the skin, vomiting and back pain.
Statistics from the National Cancer Institute estimate that 11,240 people in the U.S. will die this year as a result of multiple myeloma and that 26,850 new cases will be diagnosed.
In multiple myeloma cases, cancer cells multiply then produce an abnormal protein that displaces healthy blood cells from the bone marrow. It can cause the immune system to weaken to such a degree that patients contract other diseases of the bones or kidneys.