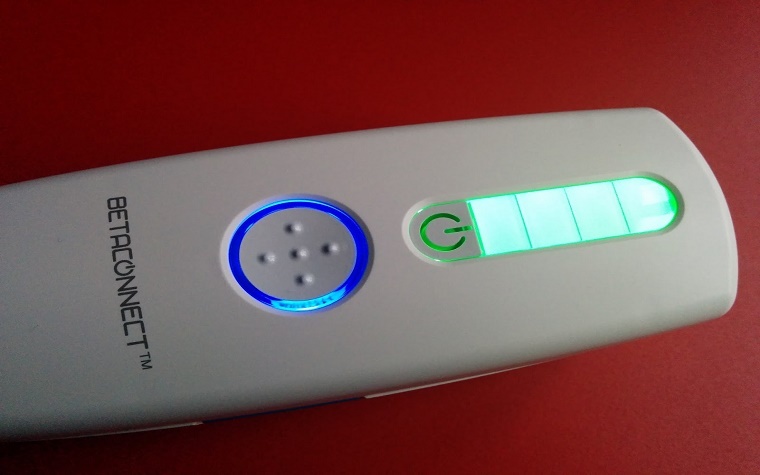
Bayer HealthCare said Sept. 25 it has received approval from the U.S. Food and Drug Administration (FDA) for Betaconnect, the first-of-its-kind electronic autoinjector indicated for the treatment of relapsing-remitting multiple sclerosis (RRMS).
The product is expected to be available in early 2016 to patients who take Betaseron (interferon beta-1b).
Betaseron is a prescription drug used to decrease the number of relapses in MS patients. It will not cure the disease, but may lessen flareups patients experience.
"Bayer has a long legacy of supporting and providing services for the RRMS community," Bayer Vice President and General Manager of Neurology Klaus Marten said. "Betaseron was the first disease-modifying therapy approved by the FDA to treat RRMS patients, and we are pleased to offer the first and only electronic auto injector for those living with the disease."
Betaconnect offers customizable injection speed and depth settings so patients can inject discreetly and with precision at the touch of a button. It also features an optional backup reminder function so patients know when it's time for their next injection.
"Offering new options to individuals with MS to help manage their disease is important since 'one size does not fit all' when considering MS treatment," Multiple Sclerosis Association of America President and CEO Douglas Franklin said.
There are approximately 400,000 people in the United States living with RRMS.