Global medical device manufacturer K2M Group Holdings Inc. commercially launched its Everest® Deformity Spinal System, an implant technology that corrects spinal deformities, Sept. 30 in the United States.
The announcement of the launch was made at the Scoliosis Research Society's (SRS) 50th Annual Meeting in Minneapolis.
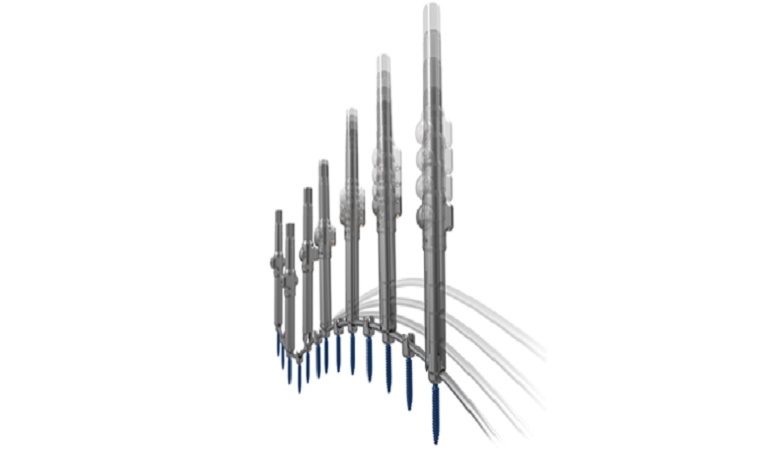
The Everest Deformity Spinal System features a top-loading pedicle screw in a variety of screw types and offers the ability to accommodate titanium and cobalt chrome rods in two different diameters, giving surgeons a variety of options during surgeries.
"The Everest Deformity system offers versatility and adaptability by providing surgeons with a variety of solutions to complex spine pathologies, including choices in rod diameter, rod material, construct configuration, and deformity correction techniques," Shay Bess, MD, chief of adult spinal deformity in the department of orthopedic surgery at the NYU Langone Medical Center's Hospital for Joint Disease in New York, said.
"The Everest Deformity system offers a streamlined and comprehensive approach and adapts to each surgeon's preferred surgical technique, thereby allowing me to make the appropriate surgical decisions for my adolescent and adult patients."
And Frank Schwab, MD, spine service chief at the Hospital for Special Surgery in New York, agreed. "The speed and control Basecamp provides exceeds my expectations and gives me the ability to reduce the rod and correct the spine in a controlled fashion," he said.
K2M President and CEO Eric Major explained that 2015 had been "a year of complex spine innovation for K2M."
"We are excited to be launching the Everest Deformity Spinal System during the 50th anniversary meeting of the Scoliosis Research Society," he said.
For more information on K2M's complete product portfolio, visit www.K2M.com.