Officials with the U.S. Food and Drug Administration (FDA) recently approved of the world’s first pacemaker that doesn’t have wired leads, designed to address heart rhythm disorders.
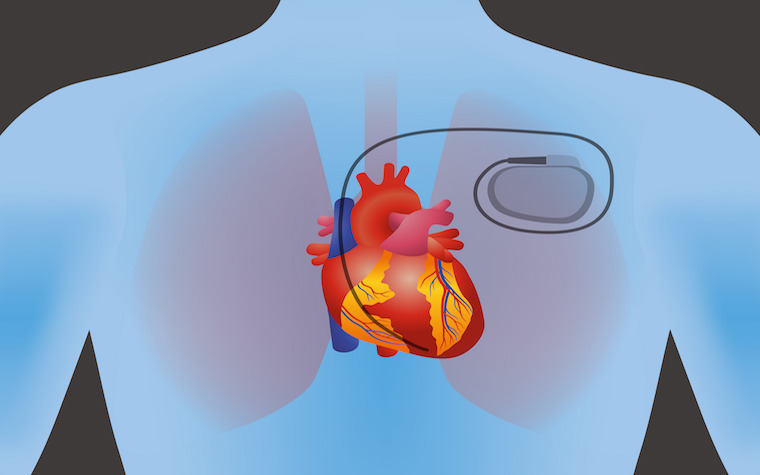
Traditional pacemakers have wired leads to make an electrical connection from the heart to the pulse-generating device. These leads are in a single chamber and connect from the pacemaker, under the skin close to the collarbone and into a vein that joins the right ventricle of the heart. This is how the leads send electrical pulses from the generator to the heart.
Studies have shown that leads can malfunction or develop infections, which then calls for a replacement device. This is why researchers have developed a new kind of pacemaker.
The new pacemaker, called the Micra Transcatheter Pacing System, is a self-contained device that measures an inch in length and sits in the right ventricle of the heart. The new pacemaker works similarly to the older model, regulating heart rates.
“As the first leadless pacemaker, Micra offers a new option for patients considering a single chamber pacemaker device, which may help prevent problems associated with the wired leads,” William Maisel, acting director of the Office of Device Evaluation at the FDA’s Center for Devices and Radiological Health, said.
Approximately one million people around the world have pacemakers implanted in their chests every year. The pacemakers are needed to create electrical impulses to remedy stalled or irregular heart beats.



