A new therapy indicated for use in treating uterine fibroids is moving into the third phase of clinical study.
Elagolix, a product of pharmaceutical company AbbVie, is the subject of a placebo study the company is conducting in collaboration with Neurocrine Biosciences Inc.
This phase will evaluate changes in menstrual blood loss after six months of treatment. The study will also assess volume of fibroids, monthly blood loss and hemoglobin levels, as well as bone mineral density.
"There are limited non-surgical treatment options for women suffering from heavy menstrual bleeding associated with uterine fibroids," Dr. Michael Severino, AbbVie's chief scientific officer, said. "AbbVie is eager to further explore Elagolix's potential to address this unmet need."
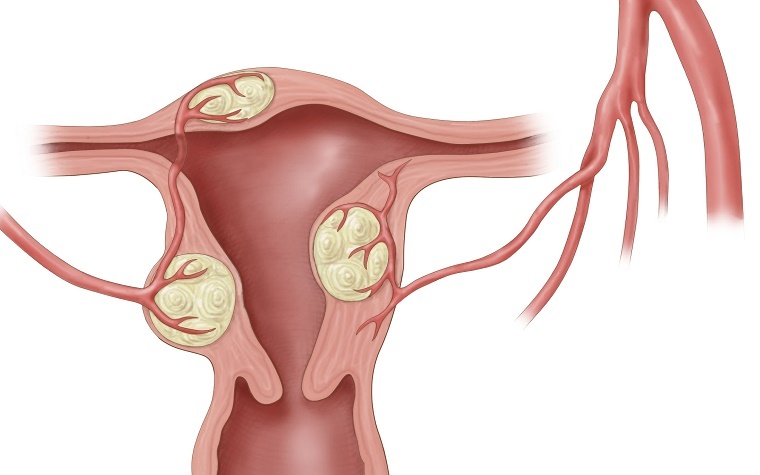
Uterine fibroids are non-cancerous masses of muscle tissue that form in the uterus. Patients most commonly diagnosed with these masses are women ages 30 to 40. The fibroids can vary greatly in size and can lead to excessive menstrual bleeding and severe menstrual pain, pain in the lower back or abdomen, painful intercourse, difficult or frequent urination, constipation or rectal pain.
Elagolix is an orally administered hormone.
This phase of the study, which will enroll about 400 subjects, will be comprised of two replicate, randomized, parallel, double-blind, placebo-controlled clinical trials.



